Calciol
By Jens Allmer
Cholecalciferol
For more technical details, read on.
Calciol
The name Cholecalciferol (aka Calciol and vitamin D) refers to a hormone that is produced in human skin when 7-Dehydrocholesterol is irradiated by UVB. Therefore, we need sufficient amounts of the molecule 7-Dehydrocholesterol and UVB. In case, we don’t get into the sun, cover large parts of our bodies with clothing, or put sunscreen on our skin, we don’t produce the human hormone Cholecalciferol aka vitamin D another name is Calciol which makes sense when considering that Calciol has one hydroxy group, Calcifediol has two and Calcitriol has three (di: 2; tri: 3; ol: signifying the addition of OH in this case).
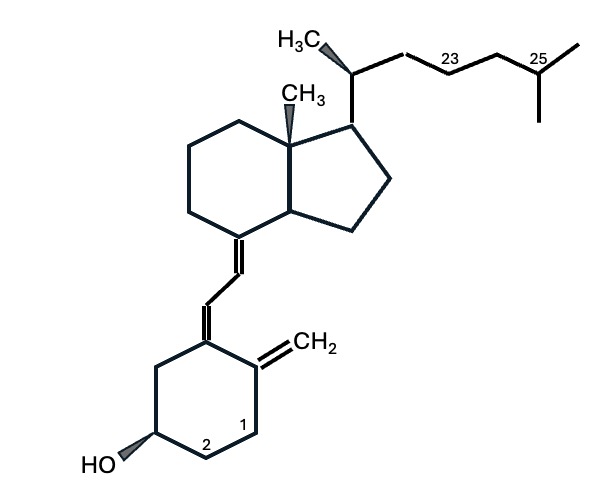 Chemical structure of Cholecalciferol
Chemical structure of Cholecalciferol
The chemical structure of Cholecalciferol (molecular weight: 385 g/mol). Some positions are numbered. Positions 1 and 25 are particularly important. When the carbon atom at position 25 is hydroxylated, another form of D3 is produced: Calcifediol. When both positions 1 and 25 are hydroxylated, the molecule is called Calcitriol, the active form of the hormone.
Cholecalciferol enters the blood either unbound via diffusion or bound to D-binding protein (DBP) when originating from the conversion of 7-dehydrocholesterol in the skin. The binding affinity of Cholecalciferol to DBP is lower than the binding affinities of Calcitriol and Calcifediol. When ingested Cholecalciferol is typically bound to chylomicrons.
Free Cholecalciferol, or chymicron-bound Cholecalciferol is readily taken up by fat and muscle cells and stored there for future usage. However, if many free DBP molecules are available, these will compete for binding and storage of Cholecalciferol will not be emphasised. Muscle and liver cells can convert Cholecalciferol to Calcifediol.
Blood levels of Cholecalciferol have not been investigated much as they are rather transient but two studies published their results: White healthy subjects (in 1984) were found to have 4 +/- 1 ng/ml whereas black healthy subjects had levels of
1 +/- 0.3 ng/ml ~ 10 +/- 3 nmol/L (Jacob et al. 1984).
In 2013 higher levels of 37 - 45 ng/ml ~ 95 - 117 nmol/L were found by (Srinivasan et al. 2013). The levels are only for reference and likely carry no biological or medical significance.
| Study | ng/ml | ng/ml | nmol/L | nmol/L |
| 1984 | 3 | 5 | 7 | 13 |
| 2013 | 37 | 45 | 95 | 117 |
Lower and higher Cholecalciferol levels found in healthy subjects in both ng/ml and nmol/L.
Many factors can influence the result. Sun exposure may not be a factor since both areas where the measurements were made are relatively sunny although we don’t know whether the measurements of Jacob et al. were made during the winter. Maybe supplementation with Vitamin D was more common in 2013.
